Insulin Resistance in the brain
Introduction
Over time, if you’re developing insulin resistance of the body, you’re also developing insulin resistance of the brain. Depending on the individual, this process of developing insulin resistance can focus primarily in the body or in the brain. Some people with insulin resistance will develop Alzheimer’s Disease, some heart disease, some Type 2 diabetes, some Cancer, some Polycystic Ovarian syndrome, etc. Why insulin resistance manifests in different ways is unclear, but it likely depends on depends on an individual’s genetics, lifestyle, environment, and other factors.
Nevertheless, there is a clear connection between insulin resistance and Alzheimer’s, so much so that in 2008 the term Type 3 Diabetes was coined as another way of referring to Alzheimer’s Disease. Alzheimer's Disease Is Type 3 Diabetes–Evidence Reviewed (Suzanne M. de la Monte, Jack R. Wands, 2008) Since 2008, there has been additional research, strengthening this link between Alzheimer’s disease (AD) and insulin resistance.
Type 2 diabetes is just one possible end result of insulin resistance. To be clear, while there is a strong relationship, one can develop Type 2 diabetes and never get Alzheimer’s. Conversely, one can have Alzheimer’s and not diabetes. One thing is clear, however, insulin resistance in the brain occurs in Alzheimer’s patients, regardless of if there is insulin resistance elsewhere in the body.
Insulin in a healthy body
Let’s review how insulin works in a normal, healthy body:
- Food, particularly sugar and starches are broken down into blood glucose (see Blood Sugar).
- The body prefers to keep glucose in the blood regulated to a near constant level (metabolic homeostasis), which is insulin’s function.
- When blood glucose rises after a meal, the pancreas releases insulin to escort the glucose out of the bloodstream into cells where it’s needed. Insulin does this by binding to receptors on the surfaces of the body’s cells. The insulin is the “key” that fits in to the receptor to “unlock” the cell and let glucose in where it is needed.
Insulin resistance in the body
If a person eats a sugary/carby meal, the body will be flooded with glucose, more than it needs. The body pumps out lots of insulin to handle all this glucose, storing it (in the form of fat), in order to get the blood sugar levels back to normal.
Over time, with lots of sugary/carby meals and snacks, this process of excess insulin production causes damage to the body: the receptors become desensitized, the insulin receptors become numb to insulin. The insulin receptors also downregulate, I other words they become fewer in number. Fat is supposed to be stored in fat cells, called adipocytes, but in most people, this is a finite capacity. When the fat cells have become overfilled they can’t get enough oxygen and they become inflamed. When there’s no more room in the fat cells, the body then looks for other places to store fat, including where fat was never meant to go: the abdominal cavity (visceral fat), other organs (liver, pancreas, kidneys), and muscle. This ectopic fat (fat stored in areas besides fat cells) interferes with cellular functions and results in organ dysfunction, Like a splinter, ectopic fat is constantly annoying/stressing the body.
A condition commonly associated with persistent high insulin is Non-alcoholic Fatty Liver disease (NAFLD). A fatty liver is insulin resistant, so it’s not regulated by insulin appropriately, it keeps pumping out glucose (gluconeogenesis) even though the body doesn’t need glucose, thus exacerbating the problem. But when the pancreas is overtaxed but able to pump out enough insulin to maintain a relatively even and low level of glucose, that’s insulin resistance in the body (simplified). Insulin resistance develops over years. Without intervention, insulin resistance ultimately becomes Type 2 Diabetes. With Type 2 Diabetes, insulin no longer works well in the body and blood sugar levels stay high. A blood sugar test alone cannot determine if a person is insulin resistant, see Blood Sugar
Insulin resistance in the brain
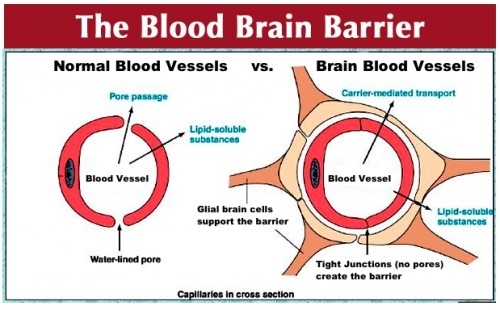
Insulin resistance in the is different than insulin resistance in the body.
In the body, glucose is escorted into cells through transporters called Glucose Transporter 4 (GLUT-4), these are regulated by insulin. But in the brain, blood vessels are surrounded by the Blood Brain Barrier (BBB). The Blood Brain Barrier adds a layer of protection for the precious brain. The endothelial cells of the BBB fit together tightly (tight junctions) so substances such as disease-causing pathogens and toxins cannot pass out of the bloodstream into the brain.
But the brain doesn’t want to impair glucose from entering. Unlike in the body, on the blood brain barrier, there are what’s called Glucose transporter 1 (GLUT-1) receptors, they let glucose into the brain without being regulated by insulin.
The brain wants unimpaired glucose passage is because the brain needs fuel and lots of it. The brain uses more energy than any other human organ. It constitutes only 3% of the body's mass, but uses up 30% of the body's energy and it needs energy 24 hours a day.
The human brain developed over millions of years under conditions that aren’t typified by today’s modern lifestyle of abundant food filled with sugar and simple carbohydrates. Rather, the brain developed under conditions of periodic food paucity, virtually no sugar, and limited high-glycemic foods. Given that past history, the brain doesn’t want to restrict access to glucose. While the brain can burn ketones and ketones burn cleaner and more efficiently, see Ketosis and Ketogenic Dietthere are some cells in the brain that can only burn glucose. The brain must have glucose.
Most of the brain’s energy consumption goes toward sustaining neurons. Because of these non-insulin dependent GLUT-1 receptors on the blood brain barrier, glucose freely flows into the brain, but with modern diets these glucose levels can be exceptionally high. The mere presence of glucose in the brain doesn’t mean the brain can use it.
The brain needs insulin. The brain is dense with insulin receptors, particularly in:
- Hippocampus (the brain’s memory center)
- Amygdala (mood)
- Cortex (cognition/executive function)
So while glucose can enter the brain without insulin, the brain can’t metabolize the glucose without insulin, and without the ability to metabolize glucose, brain cells are starved for fuel, they can’t function appropriately, ultimately they die, which is what happens when the brain is insulin resistant.
The higher one’s blood sugar, the higher one’s brain sugar, but even if the pancreas is pumping out more insulin for the body, the brain has a saturation point for insulin where it won’t allow accept any more insulin.
If you’re developing insulin resistance of the body, the brain’s insulin receptors are also becoming insulin resistant. They become damaged, desensitized, and downsize in numbers. The result of this brain insulin resistance will limit how much insulin can get into the brain. But insulin is critical for brain cells. In particular the cells of the hippocampus, (the memory center of the brain) require insulin to process the glucose. Without insulin, you have is a situation where unlimited glucose has been allowed in the brain, so the brain is swimming in a sea of glucose, yet the brain cells are literally starving to death because of insulin resistance.
To add insult to injury all that excess glucose damages the brain. It is toxic to neurons in a variety of different ways. Glucose Neurotoxicity (DR Tomlinson and NJ Gardiner, 2008)
- Depletes natural antioxidant reserves, glutathione, the body’s own antioxidant
- Promotes damaging free radical formation, these are violent molecules that create collateral damage, wrecking havoc with your DNA and more
- Generates Advanced Glycation End Products (AGEs), sticky dysfunctional proteins
- Slows nerve cell conduction speed
- Reduces growth factor activity, growth factor keeps cells healthy and robust and thriving
Too much glucose without the requisite insulin results in Cerebral Glucose Hypometabolism, which means slow glucose metabolism. This slow glucose metabolism is likely: (1) contributing to Alzheimer’s development and (2) a consequence of Alzheimer’s Disease. Can ketones compensate for deteriorating brain glucose uptake during aging? Implications for the risk and treatment of Alzheimer's disease. (SC Cunnane, et al, 2016)
In other words, there’s a vicious cycle: the slowed brain glucose uptake (hypometabolism) leads to chronic brain energy deprivation, that in turn deteriorates the neuronal function, which further diminishes the demand for glucose thereby furthering cognitive decline. This hypometabolism may begin 30 or more years before the onset of AD especially in individuals with ApoE4 genotype or maternal family history of Alzheimer's Disease.
While this cerebral glucose hypometabolism continues for decades before Alzheimer’s disease manifests, it’s not until glucose processing has diminished 15% to 25% that cognitive symptoms arise. So by the time Alzheimer’s is diagnosed, there’s already damage in the brain. Can ketones compensate for deteriorating brain glucose uptake during aging? Implications for the risk and treatment of Alzheimer's disease. (SC Cunnane, et al, 2016). This is a process that precedes Alzheimer’s Disease by decades Effects of ketone bodies in Alzheimer's disease in relation to neural hypometabolism, β‐amyloid toxicity, and astrocyte function (L Hertz, et al, 2015)



